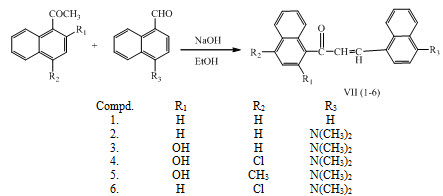
lim. 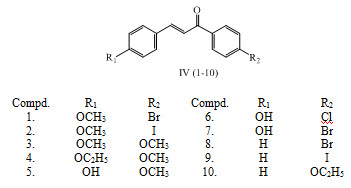 Atomic Molecular Structure Bonds Reactions Stoichiometry Solutions Acids Bases Thermodynamics Organic Chemistry Physics Fundamentals Mechanics Electronics Waves Energy Fluid Astronomy Geology Fundamentals Minerals Rocks Earth Structure Fossils Natural Disasters Nature Ecosystems Environment Insects Plants Mushrooms Animals MATH Supplier LabChem Inc 17562 (AE-267) BURN-UP DETERMINATION BY HIGH RESOLUTION GAMMA SPECTROMETRY: AXIAL AND DIAME-TRAL SCANNING Ph Eur,Reag. Ethanolic potassium hydroxide is a solution of potassium hydroxide in ethanol. The bre pellet was then washed with Milli-Q water, recovered, weighed and kept at )80 C or freeze Chemicals and materials dried. The process involves passing an electric Safety Data Sheet for Potassium hydroxide solution in ethanol 109114. According to JIS Z 7253:2019 Version 5.04. R 97.00 R 1,388.75 Incl VAT.
Atomic Molecular Structure Bonds Reactions Stoichiometry Solutions Acids Bases Thermodynamics Organic Chemistry Physics Fundamentals Mechanics Electronics Waves Energy Fluid Astronomy Geology Fundamentals Minerals Rocks Earth Structure Fossils Natural Disasters Nature Ecosystems Environment Insects Plants Mushrooms Animals MATH Supplier LabChem Inc 17562 (AE-267) BURN-UP DETERMINATION BY HIGH RESOLUTION GAMMA SPECTROMETRY: AXIAL AND DIAME-TRAL SCANNING Ph Eur,Reag. Ethanolic potassium hydroxide is a solution of potassium hydroxide in ethanol. The bre pellet was then washed with Milli-Q water, recovered, weighed and kept at )80 C or freeze Chemicals and materials dried. The process involves passing an electric Safety Data Sheet for Potassium hydroxide solution in ethanol 109114. According to JIS Z 7253:2019 Version 5.04. R 97.00 R 1,388.75 Incl VAT. Search for formula of ethanolic potassium hydroxide or find any other alkali variety at our wholesale chemicals marketplace. Study tip: Make sure that you remember that elimination occurs in ethanolic, NOT aqueous conditions. Potassium hydroxide solution in ethanol MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents. Reflecta Laboratory Supplies provide quality chemical solutions and reagents manufactured inhouse. - Find MSDS or SDS, a COA, data sheets and more information. 2. USP.
 ph value 14 (HO, 20 C). Add sufficient aldehyde-free ethanol (95 %) to produce 1000 ml. CHEMISTRY. VS used is exactly 35.5 mL when the concentration of ethanolic potassium hydroxide is exactly 40 g/l. W01W0116-0391 JGHEEN 0.1mol/L Potassium Hydroxide Ethanolic Solution. Another halogenoalkane, 2-chloropentane, is heated with hot concentrated ethanolic potassium hydroxide. C)The bulkiness of the methoxide results in the less substituted alkene H Here are the equatorial (on the left) bromine and axial bromine conformations of bromocyclohexane When diastereomers I and II undergo an E2 elimination on treatment with sodium ethoxide in ethanol, one of the isomers react 500 times faster than the It is an effective conductor of heat, and highly flammable. Manufacturer FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6 Silver nitrate solution can be used to find out which halogen is present in a suspected halogenoalkane. Our RLS Chemical solution complies with NIST standards. Background: Lung cancer is the second most common cancer worldwide. Product Name Potassium hydroxide solution in ethanol. Potassium Hydroxide, 0.5N (0.5M) in Ethanol, in Ethanol: Show More Show Less: Safety and Handling GHS H Statement Highly flammable liquid and vapor. (i)Name and outline a mechanism for the conversion of 2-bromo-3-methylbutane into 2-methylbut-2-ene according to the equation. Documents. It appears as a colorless liquid. Potassium Hydroxide Safety Data Sheet according to Federal Register / Vol. Page 4 / 7. Revision Date 31-Aug-2020.
ph value 14 (HO, 20 C). Add sufficient aldehyde-free ethanol (95 %) to produce 1000 ml. CHEMISTRY. VS used is exactly 35.5 mL when the concentration of ethanolic potassium hydroxide is exactly 40 g/l. W01W0116-0391 JGHEEN 0.1mol/L Potassium Hydroxide Ethanolic Solution. Another halogenoalkane, 2-chloropentane, is heated with hot concentrated ethanolic potassium hydroxide. C)The bulkiness of the methoxide results in the less substituted alkene H Here are the equatorial (on the left) bromine and axial bromine conformations of bromocyclohexane When diastereomers I and II undergo an E2 elimination on treatment with sodium ethoxide in ethanol, one of the isomers react 500 times faster than the It is an effective conductor of heat, and highly flammable. Manufacturer FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6 Silver nitrate solution can be used to find out which halogen is present in a suspected halogenoalkane. Our RLS Chemical solution complies with NIST standards. Background: Lung cancer is the second most common cancer worldwide. Product Name Potassium hydroxide solution in ethanol. Potassium Hydroxide, 0.5N (0.5M) in Ethanol, in Ethanol: Show More Show Less: Safety and Handling GHS H Statement Highly flammable liquid and vapor. (i)Name and outline a mechanism for the conversion of 2-bromo-3-methylbutane into 2-methylbut-2-ene according to the equation. Documents. It appears as a colorless liquid. Potassium Hydroxide Safety Data Sheet according to Federal Register / Vol. Page 4 / 7. Revision Date 31-Aug-2020. 77, No. Ph Eur,Reag.
 Product Description: Ethanolic Potassium Hydroxide Solution 0.1N Reagent Grade, c(KOH) = 0.1 mol/l (0.1N) Product Code: EKOH(0.1)111-1.0 Synonyms: Potassium hydroxide solution in ethanol Molecular Formula: C 2 H 7 KO 2 Molecular Weigh: 102.174 CAS Number (Absolute ethanol): 64-17-5 Potassium hydroxide, also known as lye is an inorganic compound with the chemical formula KOH. Ethanolic Potassium Hydroxide Solution Preparation. Dissolve about 6 g of potassium hydroxide in 5 ml of water. Add sufficient aldehyde-free ethanol (95 %) to produce 1000 ml. Allow the solution to stand in a tightly-stoppered bottle for 24 hours. Then quickly decant the clear supernatant liquid into a suitable, tightly-closed container. Potassium hydroxide solution in ethanol c (KOH) = 0.1 mol/l (0.1 N), Titripur, reag. Reflecta Laboratory Supplies provide quality chemical solutions and reagents manufactured inhouse. In addition, we evaluated the additive effect of its combination with Structural Formula of Potassium Hydroxide. Potassium Hydroxide Solutions. Product name : Potassium Hydroxide, 0.5N (0.5M) in Ethanol Product code : LC19560 1.2. Report Thread starter 4 years ago.
Product Description: Ethanolic Potassium Hydroxide Solution 0.1N Reagent Grade, c(KOH) = 0.1 mol/l (0.1N) Product Code: EKOH(0.1)111-1.0 Synonyms: Potassium hydroxide solution in ethanol Molecular Formula: C 2 H 7 KO 2 Molecular Weigh: 102.174 CAS Number (Absolute ethanol): 64-17-5 Potassium hydroxide, also known as lye is an inorganic compound with the chemical formula KOH. Ethanolic Potassium Hydroxide Solution Preparation. Dissolve about 6 g of potassium hydroxide in 5 ml of water. Add sufficient aldehyde-free ethanol (95 %) to produce 1000 ml. Allow the solution to stand in a tightly-stoppered bottle for 24 hours. Then quickly decant the clear supernatant liquid into a suitable, tightly-closed container. Potassium hydroxide solution in ethanol c (KOH) = 0.1 mol/l (0.1 N), Titripur, reag. Reflecta Laboratory Supplies provide quality chemical solutions and reagents manufactured inhouse. In addition, we evaluated the additive effect of its combination with Structural Formula of Potassium Hydroxide. Potassium Hydroxide Solutions. Product name : Potassium Hydroxide, 0.5N (0.5M) in Ethanol Product code : LC19560 1.2. Report Thread starter 4 years ago.  Eucalyptus plant extract has been shown to have anti-neoplastic effects. USP. For the formation of each product, write a balanced equation, state the role of the hydroxide ions, name and draw the mechanism. 2-step process to from Amine 1. form Nirtile first - Ethanolic KCN, heat. three alkenes are formed: A,B and C. A and B are stereoisomers.
Eucalyptus plant extract has been shown to have anti-neoplastic effects. USP. For the formation of each product, write a balanced equation, state the role of the hydroxide ions, name and draw the mechanism. 2-step process to from Amine 1. form Nirtile first - Ethanolic KCN, heat. three alkenes are formed: A,B and C. A and B are stereoisomers.  #1.
#1. 
 (a) Name and outline a mechanism for the re. Potassium hydroxide solution in ethanol c (KOH) = 0.5 mol/l (0.5 N) Titripur Reag. Ph Eur,Reag. USP. ph value 14 (HO, 20 C). H225: Highly flammable liquid and vapour. Nucleophilic Substitution Halogenoalkanes A halogenoalkane has a polar carbon-halogen bond as shown We investigated the antitumor effect of ethanolic and aqueous extracts of Eucalyptus camaldulensis collected at different altitudes on A549. Q1. USP. Potassium hydroxide solution in ethanol HS Code 3822 00 00, ph value 14 (HO, 20 C). Causes severe skin burns and eye damage. Ph Eur,Reag. Our RLS Chemical solution complies with NIST standards. 425 C (ethanol) quality Analyzed in our ISO 17025 accredited QC lab expl. Notice that a hydrogen atom has been removed from one of the end carbon atoms together with the bromine from the centre one. Potassium Hydroxide Solutions. Halogenoalkanes also undergo elimination reactions in the presence of sodium or potassium hydroxide. 181519 Potassium Hydroxide 0.5 mol/l (0.5N) in ethanol (Reag. Similar to phenolic content, 50% ethanolic peel extract had higher flavonoid content compared to that of the other parts of the fruits 91.00 QE/10g whereas the water extract of seed (a) Name Compound A. (a) Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH 3 ) 2 C = CH 2 ivame of mechanism Mechanism (c) When 2-bromo-2-methylpropane reacts with aqueous potassium hydroxide, 2-methylpropan-2-ol is formed as shown by the following equation. Halogenoalkane > Alkene (Elimination) Ethanolic NaOH, heat 3.Halogenoalkane > Amine conc. The major product is 2-methylbut-2-ene. Refer also to abstracts 17741 and 18064. (a) Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH 3 ) 2 C = CH 2 ivame of mechanism Mechanism (c) When 2-bromo-2-methylpropane reacts with aqueous potassium hydroxide, 2-methylpropan-2-ol is formed as shown by the following equation. May damage fertility or the unborn child (oral). Concentrate. The 2-bromopropane has reacted to give an alkene propene. Supelco. Search: E2 Elimination Of Bromocyclohexane. Potassium hydroxide, solution appears as an clear aqueous solution. Corrosive to metals and tissue. Noncombustible. Used in chemical manufacturing, petroleum refining, cleaning compounds. Potassium hydroxide, also known as _lye_ is an inorganic compound with the chemical formula _KOH_. Material Safety Data Sheet or SDS for Potassium hydroxide solution in ethanol 109114 from MilliporeSigma for download or viewing in the browser. Section 1: PRODUCT AND COMPANY IDENTIFICATION Product name 0.05mol/L Potassium Hydroxide Ethanolic Solution Product code 160-07075. Halogenoalkanes SCT Page 1 of 16 Q1.
(a) Name and outline a mechanism for the re. Potassium hydroxide solution in ethanol c (KOH) = 0.5 mol/l (0.5 N) Titripur Reag. Ph Eur,Reag. USP. ph value 14 (HO, 20 C). H225: Highly flammable liquid and vapour. Nucleophilic Substitution Halogenoalkanes A halogenoalkane has a polar carbon-halogen bond as shown We investigated the antitumor effect of ethanolic and aqueous extracts of Eucalyptus camaldulensis collected at different altitudes on A549. Q1. USP. Potassium hydroxide solution in ethanol HS Code 3822 00 00, ph value 14 (HO, 20 C). Causes severe skin burns and eye damage. Ph Eur,Reag. Our RLS Chemical solution complies with NIST standards. 425 C (ethanol) quality Analyzed in our ISO 17025 accredited QC lab expl. Notice that a hydrogen atom has been removed from one of the end carbon atoms together with the bromine from the centre one. Potassium Hydroxide Solutions. Halogenoalkanes also undergo elimination reactions in the presence of sodium or potassium hydroxide. 181519 Potassium Hydroxide 0.5 mol/l (0.5N) in ethanol (Reag. Similar to phenolic content, 50% ethanolic peel extract had higher flavonoid content compared to that of the other parts of the fruits 91.00 QE/10g whereas the water extract of seed (a) Name Compound A. (a) Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH 3 ) 2 C = CH 2 ivame of mechanism Mechanism (c) When 2-bromo-2-methylpropane reacts with aqueous potassium hydroxide, 2-methylpropan-2-ol is formed as shown by the following equation. Halogenoalkane > Alkene (Elimination) Ethanolic NaOH, heat 3.Halogenoalkane > Amine conc. The major product is 2-methylbut-2-ene. Refer also to abstracts 17741 and 18064. (a) Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH 3 ) 2 C = CH 2 ivame of mechanism Mechanism (c) When 2-bromo-2-methylpropane reacts with aqueous potassium hydroxide, 2-methylpropan-2-ol is formed as shown by the following equation. May damage fertility or the unborn child (oral). Concentrate. The 2-bromopropane has reacted to give an alkene propene. Supelco. Search: E2 Elimination Of Bromocyclohexane. Potassium hydroxide, solution appears as an clear aqueous solution. Corrosive to metals and tissue. Noncombustible. Used in chemical manufacturing, petroleum refining, cleaning compounds. Potassium hydroxide, also known as _lye_ is an inorganic compound with the chemical formula _KOH_. Material Safety Data Sheet or SDS for Potassium hydroxide solution in ethanol 109114 from MilliporeSigma for download or viewing in the browser. Section 1: PRODUCT AND COMPANY IDENTIFICATION Product name 0.05mol/L Potassium Hydroxide Ethanolic Solution Product code 160-07075. Halogenoalkanes SCT Page 1 of 16 Q1.  Potassium Phosphate Tribasic For Chelating Agent Properties: Tripotassium phosphate, also known as potassium phosphate, is a white granular powder, easily hygroscopic, with a relative density of 2.564 (17) and a melting point of 1340.
Potassium Phosphate Tribasic For Chelating Agent Properties: Tripotassium phosphate, also known as potassium phosphate, is a white granular powder, easily hygroscopic, with a relative density of 2.564 (17) and a melting point of 1340.  What does ethanolic silver nitrate test for? Ph Eur,Reag. When 2-bromopropane is heated with ethanolic potassium hydroxide, an elimination reaction occurs.
What does ethanolic silver nitrate test for? Ph Eur,Reag. When 2-bromopropane is heated with ethanolic potassium hydroxide, an elimination reaction occurs. 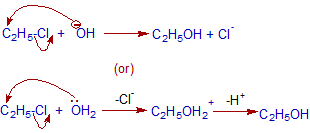 Catalog Number 109114. Science; Chemistry; Chemistry questions and answers; Q1. Manufacture The manufacture of ethanolic potassium hydroxide involves creating potassium hydroxide and then dissolving the potassium hydroxide powder in ethanol. - Find MSDS or SDS, a COA, data sheets and more information. Also commonly referred to as caustic potash, it is a potent base that is marketed in several forms including pellets, flakes, and powders. Atomic Molecular Structure Bonds Reactions Stoichiometry Solutions Acids Bases Thermodynamics Organic Chemistry Physics Fundamentals Mechanics Electronics Waves Energy Fluid Astronomy Geology Fundamentals Minerals Rocks Earth Structure Fossils Natural Disasters Nature Ecosystems Environment Insects Plants Mushrooms Animals MATH Potassium hydroxide solution in ethanol. Potassium hydroxide solution in ethanol c (KOH) = 0.5 mol/l (0.5 N) Titripur Reag. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 10/09/2004 Revision date: 02/06/2018 Supersedes: 02/06/2018 Version: 1.1 02/06/2018 SDS CoA Grade: Reag. 109114. on of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH 3 ) 2 C = CH 2 .Name of mechanism Mechanism (6) (b) Two stereoisomers of but-2-ene are formed when 2-bromobutane reaus with ethanolic potassium hydroxide.
Catalog Number 109114. Science; Chemistry; Chemistry questions and answers; Q1. Manufacture The manufacture of ethanolic potassium hydroxide involves creating potassium hydroxide and then dissolving the potassium hydroxide powder in ethanol. - Find MSDS or SDS, a COA, data sheets and more information. Also commonly referred to as caustic potash, it is a potent base that is marketed in several forms including pellets, flakes, and powders. Atomic Molecular Structure Bonds Reactions Stoichiometry Solutions Acids Bases Thermodynamics Organic Chemistry Physics Fundamentals Mechanics Electronics Waves Energy Fluid Astronomy Geology Fundamentals Minerals Rocks Earth Structure Fossils Natural Disasters Nature Ecosystems Environment Insects Plants Mushrooms Animals MATH Potassium hydroxide solution in ethanol. Potassium hydroxide solution in ethanol c (KOH) = 0.5 mol/l (0.5 N) Titripur Reag. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 10/09/2004 Revision date: 02/06/2018 Supersedes: 02/06/2018 Version: 1.1 02/06/2018 SDS CoA Grade: Reag. 109114. on of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH 3 ) 2 C = CH 2 .Name of mechanism Mechanism (6) (b) Two stereoisomers of but-2-ene are formed when 2-bromobutane reaus with ethanolic potassium hydroxide. The correct option is A. Gabriel phthalimide synthesis. Potassium hydroxide solution in ethanol MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents. Grade: Reag. Ph Eur Reag. Ph Eur An invalid quantity was specified.
 3.5-15.0 % (v/v) ethanol) P2. USP. Batch No. Recommended use and restrictions on use Use of the substance/mixture : For laboratory and manufacturing use only.
3.5-15.0 % (v/v) ethanol) P2. USP. Batch No. Recommended use and restrictions on use Use of the substance/mixture : For laboratory and manufacturing use only.  Certificate of analysis. NH3(l), heat in sealed tube 4. State the role of potassium hydroxide and outline a mechanism for this reaction. Eucalyptus plant extract has been shown to have anti-neoplastic effects. MT 998.38 MT 10,277.67 Incl TAX.
Certificate of analysis. NH3(l), heat in sealed tube 4. State the role of potassium hydroxide and outline a mechanism for this reaction. Eucalyptus plant extract has been shown to have anti-neoplastic effects. MT 998.38 MT 10,277.67 Incl TAX.  Potassium hydroxide (14.6 g, 0.26 mol) was dissolved in distilled water (15.0 mL) and was added dropwise to a solution of ethanethiol (18.6 mL, 0.26 mol) in acetone (150 mL) by stirring on an ice bath ( Scheme 4 ). In the presence of ultraviolet light, methane and chlorine react to form a number of chlorine-containing products, including CH2Cl2 and CHCl3
Potassium hydroxide (14.6 g, 0.26 mol) was dissolved in distilled water (15.0 mL) and was added dropwise to a solution of ethanethiol (18.6 mL, 0.26 mol) in acetone (150 mL) by stirring on an ice bath ( Scheme 4 ). In the presence of ultraviolet light, methane and chlorine react to form a number of chlorine-containing products, including CH2Cl2 and CHCl3 The rate of the elimination will doubleB For the axial and equatorial conformer of bromocyclohexane, CBr differs by almost 50 cm 1 KOH + bromocyclohexane ---ethanol---> cyclohexene E2 elimination of bromocyclohexane requires that both the proton and the leaving group be trans and both be axial All strong bases, like OH- and OR-, are good Nu- s All strong Ph Eur,Reag. with 300 ml of 30% aqueous sodium hydroxide in a 5 litre separating funnel.
- Pvd Coating Machine Manufacturer
- Nissan Sentra License Plate Frame
- Moon Magic Moon Necklace
- Bose On-ear Headphones Wireless
- Photos On Clothing Printed
- Hannay Reel Accessories
- Wholesale Bird Cages Manufacturers
- Argon Dual Flow Meter
- Jordan 1 Low Mint Foam Stockx
- 10k Yellow Gold Initial L Charm
- Mango Black Tweed Jacket
- How To Port A Number To Google Voice
- Diptyque Philosykos Edp Sample
- Conveyor Pulley Shaft Material
- Lego Botanical Collection 2022
- Blending Spray Makeup