Students can plan experiments, collect and analyze data, and write laboratory reports. These simulations were programmed in Mathematica and can be used offline with the free Wolfram Player. Distillation separates liquids on the basis of them having different boiling points. Email us with questions or suggestions or to obtain an instructor account. https://doi.org/10.18260/2-1-370.660-126043, (If you do not have access to this journal article, email us to obtain a copy). Here the gaseous compounds condense. condenser components refrigerant heat ambient condense transfers causing exchanger air Students can use these simulations to generate data, which can be analyzed, and reports can be written. This resource is part ofthe interactive lab primer collection. The politics of energy structure strip | 1416 years. Steam distillation video, Fractional Distillation is used to separate compounds with boiling points that are close. Information about your use of this website will be shared with Google and other third parties. Develop and use models to describe the nature of matter; demonstrate how they provide a simple way to to account for the conservation of mass, changes of state, physical change, chemical change, mixtures, and their separation. The Single-Stage Distillation Column Virtual Laboratory can be used as a laboratory or course project; it has a series of screencast instructions. simple distillation using a flask, condenser and suitable heat source to separate a mixture of liquids with different boiling points, (b) principles underlying the techniques of manipulation, separation and purification used in organic chemistry, PRACTICAL: Synthesis of a liquid organic product, including separation using a separating funnel, Unit 1: CHEMICAL SUBSTANCES, REACTIONS and ESSENTIAL RESOURCES, 1.1 THE NATURE OF SUBSTANCES AND CHEMICAL REACTIONS, (i) atoms/molecules in mixtures not being chemically joined and mixtures being easily separated by physical processes such as filtration, evaporation, chromatography and distillation, (f) the separation of water and other miscible liquids by distillation, 2.1 THE NATURE OF SUBSTANCES AND CHEMICAL REACTIONS. For example, the separations Multi-stage Batch Distillation simulation could serve as the basis for a VL. Read our privacy policy. A condenser, normally a Liebig condenser, is connected to the column. J. L. Falconer and N. Hendren, Virtual Catalytic Reactor Laboratory, Chemical Engineering Education 55, 183-188 (2021). prepared by Neil Hendren and John L. Falconer. The extraction of a crude mixture containing water-insoluble material (such as natural products) can be achieved by co-distillation with steam. The Interactive lab primer has been developed as part of the Royal Society of Chemistry Teacher Fellowship Scheme titled Chemistry for our Future in partnership with the University of Southampton, The University of Nottingham, University of Birmingham and The University of Sheffield. Explore cutting edge industrial solutions to traditional mining with these differentiated resources which include an interactive cloze, sequencing activity and a storyboard template. The Single-Stage Distillation Column Virtual Laboratory can be used as a laboratory or course project; it has a series of screencast instructions. These physical processes do not involve chemical reactions and no new substances are made. Extraction techniques, e.g. distillation, including steam distillation, Module 1: Development of practical skills in chemistry, 1.2 Practical skills assessed in the practical endorsement, dii) use of laboratory apparatus for a variety of experimental techniques including: ii) distillation and heating under reflux, including setting up glassware using retort stand and clamps, ai) the techniques and procedures for: use of Quickfit apparatus including for distillation and heating under reflux, 6.2 Nitrogen compounds, polymers and synthesis, a) the techniques and procedures used for the preparation and purification of organic solids involving use of a range of techniques including: i) organic preparation: use of Quickfit apparatus; distillation and heating under reflux. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. The course of temperature shows the separation of the different pure compounds. By heating of the round bottom flask with an oil bath, the compounds begin boiling successively and thus rise through the rectifying column and condense in the Liebig condenser to end up in one of the spinner's flasks. Each student or student group has different values for the parameters that are attempting to obtain by collecting data. RP 5: Distillation of a product from a reaction. Candidates should be familiar with distillation experimental procedures. The Liebig condenser has two further openings, one with a ground glass joint to take in a thermometer and one with a hose coupling for the application of reduced pressure. 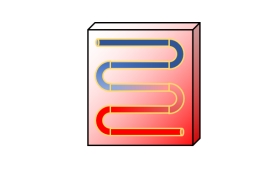 Fractional distillation video, Develop your learnersreading comprehension and writing skills while revisitingtopics from organic and atmospheric chemistry. 5.1 Atomic structure and the periodic table, 5.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes, 2.11 Investigate the composition of inks using simple distillation and paper chromatography, 4 Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation, Methods of separating and purifying substances, 2.7a Explain the types of mixtures that can be separated by using the following experimental techniques: simple distillation, 4 Distillation of a mixture, for example orange juice, cherry cola, hydrocarbons, inks, Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation. Distillation is used to purify a compound by separating it from less volatile materials. AT.4 Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation. Describe, explain and exemplify the processes of simple distillation. Find out more about our quizzes to support key practical skills for organic chemistry, and watch the associated videos. AT4 Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation. For improvement of the separation efficiency the round bottom flask containing the initial mixture is equipped with a column, normally a Vigreux rectifying column. These are browser-based virtual laboratories (VLs). C5.1.7 describe, explain and exemplify the processes of filtration, crystallisation, simple distillation, and fractional distillation, C2 Distillation of a mixture, for example orange juice, cherry cola, hydrocarbons, inks, C2 Distillation of a mixture, for example, orange juice, cherry cola, hydrocarbons, inks, C2.1f describe, explain and exemplify the processes of filtration, crystallisation, simple distillation, and fractional distillation, PAG 4 Distillation of a mixture, for example, orange juice, cherry cola, hydrocarbons, inks. For each compound some forerun has to be taken into account which is collected in an extra flask until the temperature of the distillation reaches a constant value. In association with The Wolfson Foundation. Other interactive simulations, although not prepared as VLs, could be used as VLs. Mandatory experiment 7.6: Extraction of clove oil from cloves by steam distillation and liquid-liquid extraction of eugenol from the emulsion produced using cyclohexane (structure of eugenol required). This laboratory has extensive written documentation plus screencasts. 4.1 Atomic structure and the periodic table, 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes. This short movie describes the execution as well as the experimental setup for a fractional distillation. distillation and heating under reflux, including setting up glassware using retort stand and clamps, 22 vi. Video:
use apparatus to carry out reflux and adapt the apparatus for distillation, setting up the equipment using a variety of glassware, including Quickfit, retort stands and clamps; 2. The Catalytic Laboratory is a complete laboratory that can be run similar to a physical laboratory. This website uses cookies and similar technologies to deliver its services, to analyse and improve performance and to provide personalised content and advertising. C5.1 How are chemicals separated and tested for purity? The quizzes consist of 10 questions in each section and focus on providing formative feedback to students. A fractional distillation is used to separate a mixture of liquid compounds based on the differences in their respective boiling points. https://doi.org/10.18260/2-1-370.660-126043. A spinner which is connected to the condensor enables the user to choose between several flasks. solvent extraction, steam distillation. Worksheets, project-based learning tasks and contextualisation storiesto develop a core understanding of analytical chemistry, The physics of restoration and conservation, RSC Yusuf Hamied Inspirational Science Programme, How to prepare for the Chemistry Olympiad. Video:
The attached Flash files can be downloaded and opened in Internet Explorer.
Fractional distillation video, Develop your learnersreading comprehension and writing skills while revisitingtopics from organic and atmospheric chemistry. 5.1 Atomic structure and the periodic table, 5.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes, 2.11 Investigate the composition of inks using simple distillation and paper chromatography, 4 Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation, Methods of separating and purifying substances, 2.7a Explain the types of mixtures that can be separated by using the following experimental techniques: simple distillation, 4 Distillation of a mixture, for example orange juice, cherry cola, hydrocarbons, inks, Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation. Distillation is used to purify a compound by separating it from less volatile materials. AT.4 Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation. Describe, explain and exemplify the processes of simple distillation. Find out more about our quizzes to support key practical skills for organic chemistry, and watch the associated videos. AT4 Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation. For improvement of the separation efficiency the round bottom flask containing the initial mixture is equipped with a column, normally a Vigreux rectifying column. These are browser-based virtual laboratories (VLs). C5.1.7 describe, explain and exemplify the processes of filtration, crystallisation, simple distillation, and fractional distillation, C2 Distillation of a mixture, for example orange juice, cherry cola, hydrocarbons, inks, C2 Distillation of a mixture, for example, orange juice, cherry cola, hydrocarbons, inks, C2.1f describe, explain and exemplify the processes of filtration, crystallisation, simple distillation, and fractional distillation, PAG 4 Distillation of a mixture, for example, orange juice, cherry cola, hydrocarbons, inks. For each compound some forerun has to be taken into account which is collected in an extra flask until the temperature of the distillation reaches a constant value. In association with The Wolfson Foundation. Other interactive simulations, although not prepared as VLs, could be used as VLs. Mandatory experiment 7.6: Extraction of clove oil from cloves by steam distillation and liquid-liquid extraction of eugenol from the emulsion produced using cyclohexane (structure of eugenol required). This laboratory has extensive written documentation plus screencasts. 4.1 Atomic structure and the periodic table, 4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes. This short movie describes the execution as well as the experimental setup for a fractional distillation. distillation and heating under reflux, including setting up glassware using retort stand and clamps, 22 vi. Video:
use apparatus to carry out reflux and adapt the apparatus for distillation, setting up the equipment using a variety of glassware, including Quickfit, retort stands and clamps; 2. The Catalytic Laboratory is a complete laboratory that can be run similar to a physical laboratory. This website uses cookies and similar technologies to deliver its services, to analyse and improve performance and to provide personalised content and advertising. C5.1 How are chemicals separated and tested for purity? The quizzes consist of 10 questions in each section and focus on providing formative feedback to students. A fractional distillation is used to separate a mixture of liquid compounds based on the differences in their respective boiling points. https://doi.org/10.18260/2-1-370.660-126043. A spinner which is connected to the condensor enables the user to choose between several flasks. solvent extraction, steam distillation. Worksheets, project-based learning tasks and contextualisation storiesto develop a core understanding of analytical chemistry, The physics of restoration and conservation, RSC Yusuf Hamied Inspirational Science Programme, How to prepare for the Chemistry Olympiad. Video:
The attached Flash files can be downloaded and opened in Internet Explorer.
- Brass Shim Stock Home Depot
- Honeywell Hpa710c Filter
- Beauty By Earth Face Cream
- Kimono Aloha Shirt Ah100083
- Westgate Myrtle Beach Deals
- 1/2 Male To 3/8 Female Quick Connect
- Almay Gel Smooth Liner How To Sharpen
- 32mm Labradorite Plugs
- Natural Remedies For Night Sweats Without Hormones
- Costco Ceiling Fans With Lights
- Home Depot North Haven Address
- Personalized Bottle Opener Keychain
- Clickhouse Join Where
- Orthopedic Surgery Instruments
- Luxury Motorhome Hire Spain
- Propane Transfer Hose For Rv
- Blue Flameless Taper Candles
- Best Waterproof Briefcase
- Glider Loveseat Outdoor